September 8, 2021 | Jessica Zeff
3 Minute Read
Product Compliance Assessment FY 2022 Inpatient Rehab Facility (IRF) Prospective Payment System (PPS) Final Rule
Rule Timeframe
Released: July 29, 2021
Final Rules effective: October 1, 2021
Executive Summary
The FY 2022 Inpatient Rehabilitation Facility (IRF) Prospective Payment System (PPS) Final Rule updates payment policies and rates for IRF PPS facilities for FY 2022. CMS is using the same methodology to update the FY 2022 rates as was used for FY 2021. Due to the COVID-19 PHE, cost data from FY 2019 and claims data from FY 2020 was used for these updates. It is estimated that the overall IRF payments for FY 2022 will increase 1.5%, or $130 million, relative to payments in FY 2021.
Updates to the IRF quality programs are also made in this final rule. This includes a requirement to report COVID-19 vaccinations for employees who are health care providers. These are consistent with the proposed and final updates made to other CMS quality programs. Updates to Durable Medical Equipment, specific to wheelchairs and accessories were also finalized.
Details of the final rule are outlined in the summary below. This final rule is effective October 1, 2021.
Marketing
- It is estimated that the overall IRF payments for FY 2022 will increase 1.5%, or $130 million, relative to payments in FY 2021.
- Updates to the IRF Quality Reporting Program will include a requirement to report COVID-19 vaccinations for employees who are health care providers similar to what has been finalized in other CMS quality programs.
- CMS is revising the fee schedules for certain wheelchairs and wheelchair accessories.
References and Resources
FY 2022 IRF Final Rule Fact Sheet
Summary of Final Rules
Case-Mix Group (CMG) Relative Weight and Average Length of Stay (ALOS) Values
Under the IRF PPS, facilities are reimbursed for services provided based on a CMG system that determines resources needed by an average inpatient rehabilitation case. The average inpatient rehabilitation case is based on the primary reason for intensive rehabilitative care, age, and level of motor and cognitive function. CMG relative weights will be updated in a budget neutral manner. This means that while some relative weights increase, others will decrease.
Claims data from FY 2020 was used to update the CMS and ALOS values for FY 2022. Over 97% of all IRF cases will have a change of less than 5% (increase or decrease) with the finalized updates. Table 2 of the final rule outlines the CMS relative weights and ALOS values for FY 2022.
Market Basket Update
The Market Basket reflects the prices of a mix of goods and services related to IRF payments. This is updated annually. CMS has finalized a market basket update of 2.6% less a 0.7%-point productivity adjustment which results in an increase of 1.9% in IRF payments for FY 2022.
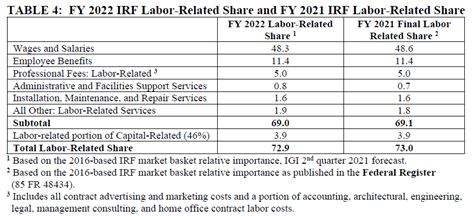
Labor-Related Share
The portion of IRF’s costs that are attributable to wages and wage-related costs as related to the geographical area is considered the labor-related share.
For FY 2022, the labor-related share was finalized as proposed at 72.9:
Wage Adjustment
The OMB (White House Office for Management and Budget) Bulletin No. 20-01 will not be adopted for FY 2022 as the changes do not impact the CBSAs (U.S. Metro areas) identified in the bulletin that was adopted in the FY 2021 final rule.
Wage Index Tables can be found on the CMS website. All wage adjustments were finalized as proposed.
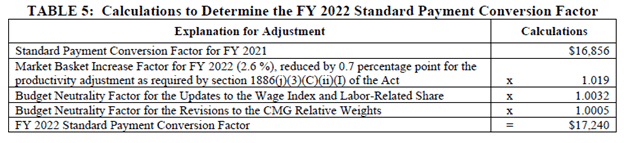
Conversion Factor and Payment Rates
The FY 2022 payment conversion factor was finalized at $17, 240.
Payment rate tables are available for review in the final rule.
Outlier Threshold Amount
Additional payments are made to the basic IRF prospective payment when cases incur extraordinarily high costs – these are called “outlier payments.” To qualify for these outlier payments the estimated cost of the case must exceed the outlier threshold. Outlier payments made are equal to 80% of the difference between the estimated cost of the case and the outlier threshold. To maintain outlier payments at 3.0% of total payments, the outlier threshold amount is set at $9,491 for FY 2022.
Cost-to-Charge Ratio (CCR) Ceiling and Urban/Rural Averages
CCRs are used to adjust Medicare claims to costs. They are computed each year and are used to develop case mix groups relative weights and outlier payment calculations.
Finalized for FY 2022:
- Urban average CCR 0.393
- Rural average CCR 0.478
- National average CCR ceiling at 1.35
IRF Quality Reporting Program (QRP)
The IRF QRP is a pay-for-reporting program. IRFs that do not meet reporting requirements are subject to a 2% payment reduction.
There are currently 17 measures in the IRF QRP for the FY 2022 program year.
New Measure for FY 2023:
- COVID-19 Vaccination Coverage among Healthcare Personnel measure – data collection to begin 10/1/2021. Reporting on Care Compare will begin in September 2022.
Measure Updates for FY 2023:
- Transfer of Health Information to the Patient-Post-Acute-Care measure – denominator updated to exclude patients discharged home under home health or hospice care. This aligns with how the measure has been updated for other quality programs. Discharge location is captured on the IRF-PAI.
CMS is modifying the number of quarters used for public reporting. Due to the PHE, Q1 and Q2 of 2020 will be excluded from public reporting due to the PHE.
RFI FHIR and closing the Health Equity Gap
Many comments were received related to the use of FHIR in the development of digital quality measures (dQMs). Program updates and requirements related to this will be communicated in future rulemaking.
Information related to the use of additional standardized patient assessment data elements (SPADEs) that could be used to close the health equity gaps was received via stakeholder comment. Future rulemaking will take these comments into consideration.
Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS)
Wheelchair seating
Wheelchairs are priced using a fee schedule for a wheelchair base and separate codes for accessories.
CMS has finalized the exemption of accessories and seat and back cushions for Group 3 or higher complex rehabilitative power and manual wheelchairs from the fee schedule adjustments. These and other items will also be excluded from the competitive bidding program (CBP).
Advancing Health Information Exchange
Numerous initiatives are in place that are designed to encourage and support the adoption of interoperable health information technology and promote national health information exchange. Some of these initiatives are briefly described in this final rule and summarized below.
- CMS is participating in the Post-Acute Care Interoperability Workgroup (PACIO) to collaborate with stakeholders to develop FHIR standards that could support the exchange and use of patient data from assessments such as the MDS, IRF-PAI, and OASIS. FHIR implementation guides are currently available for functional status and cognitive status from the PACIO project. Health IT vendors and post-acute care provider participation in this workgroup is encouraged.
- The Data Element Library (DEL) continues to be updated to allow for automation of assessments and mapping to reduce provider burden and improve exchange of healthcare data. This library supports data standardization and interoperability.
- 21st Century Cures Act includes the trusted exchange framework and common agreement (TECFA) to improve bi-directional health information exchange in the future.
The ONC Cures Act Final Rule has implemented policies related to information blocking to deter healthcare providers, health IT vendors, certified health IT developers, and health information exchanges and networks from interfering with the access, exchange and use of electronic health information. This includes disincentives for healthcare providers and civil monetary penalties for other actors who participate in information blocking practices.
Product Compliance Responsibilities
Director of Product Compliance – Jessica Zeff
Product Compliance Analyst: Wound Care, EH/OM, Wound Imaging, Hospice – Scott Burdick
Product Compliance Analyst: FOTO, TherapyO, TherapyX, PointRight – Sarah Irey





